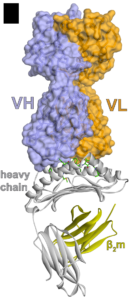
Figure 1: Overview of the TCRm-AFP/HLA-A*02 complex with the heavy and light chains of the TCRm
Emeryville, California, August 3, 2022 — Eureka Therapeutics, Inc., a clinical-stage biotechnology company developing novel T cell therapies to treat solid tumors, today announced the publication of a study in Nature’s Scientific Reports entitled “Validation and promise of a TCR mimic antibody for cancer immunotherapy of hepatocellular carcinoma”. The study was led by Dr. Cheng Liu, President and Chief Executive Officer of Eureka, Dr. Brian M. Baker and Dr. Moumita Dasgupta of the University of Notre Dame, and Dr. Chang Liu of The First Affiliated Hospital of Xi’an Jiaotong University.
T cell receptor mimic (TCRm) antibodies represent a novel approach to address one of the limitations of immunotherapy. Whereas traditional therapeutics antibodies are limited to targeting cell surface antigens, TCRm antibodies can target intracellular antigens presented by cell surface major histocompatibility complex (MHC) proteins, which enables the targeting of otherwise undruggable cancer antigens.
Alpha fetoprotein (AFP) is an intracellular antigen found in hepatocellular carcinoma (HCC), the predominant type of liver cancer. In a previous study, Eureka reported that an engineered TCRm targeting alpha-fetoprotein (AFP)-MHC complex can effectively redirect CAR-T cells against liver cancer cells.
In this study, the crystal structure showed the AFP-MHC targeting TCRm antibody binding directly over the center of the HLA protein. Unlike natural TCRs, the TCRm antibody interfaced with the AFP/HLA-A*02 complex by engaging the AFP peptide along its entire length, contacting most of the amino acids and interacting rigidly with the target complex. The significant interactions with the peptide likely confers the high affinity and specificity of the TCRm to AFP/HLA-A*02 observed in vitro and in animal studies when compared to natural TCRs. Moreover, no off-target effects were observed in the pre-clinical studies.
“TCR mimic antibodies represent a novel way to target the subset of cancer antigens found intracellularly,” said Dr. Brian M. Baker, Coleman Professor of Life Sciences at the University of Notre Dame. “The details seen in the structural and biochemical data and the resulting high specificity and affinity for this TCRm could address some of the previous safety concerns about non-specific or off-target recognition in humans.”
Eureka fused the binding domain of the TCRm to the γ and δ subunits of a TCR, and co-expressed a co-stimulatory molecule to create its proprietary ARTEMIS® T cell receptor. The engineered T cells showed potent killing activity against AFP-positive cancer cell lines in vitro and in vivo, with no off-target reactivity observed.
A first-in-human safety assessment of anti-AFP targeting ARTEMIS® T cells was conducted over a 17-month period on 6 HCC patients at the First Affiliated Hospital of Xi’an Jiaotong University in China. The anti-AFP targeting ARTEMIS® T cells demonstrated a favorable safety profile with no significant treatment-related adverse events. T cell expansion after infusions and a commensurate drop in serum AFP were detected in most patients in this study.
Eureka is currently conducting two clinical trials (ARYA-1 and ARYA-2) in the United States targeting AFP in patients with liver cancer using TCRm antibodies engineered onto ARTEMIS® T cells (ET140203). A third trial (ARYA-3) targets the GPC3 protein, also found on liver cancer cells, with ARTEMIS® T cells (ECT204). All three trials were granted Orphan Drug Designation by the U.S. Food and Drug Administration. For more information, visit www.eurekaconnectme.com.
ABOUT EUREKA THERAPEUTICS, INC.
Eureka Therapeutics, Inc. is a privately held clinical-stage biotechnology company focused on developing novel T cell therapies to treat cancers. Its core technology centers around its proprietary ARTEMIS® cell receptor platform and E-ALPHA® antibody discovery platform for the discovery and development of potentially safer and more effective T cell therapies for the treatment of solid tumors and hematologic malignancies. The company currently has two clinical programs, ET140203 (ARYA1 for adults and ARYA2 for pediatrics) and ECT204 (ARYA3), in Phase I/II US trials in patients with advanced liver cancer.
Eureka Therapeutics, Inc. is headquartered in the San Francisco Bay Area. For more information on Eureka, please visit www.eurekatherapeutics.com.
CONTACTS:
Eureka Therapeutics, Inc.
Natalie Liu
Investor Relations
510-318-9215


